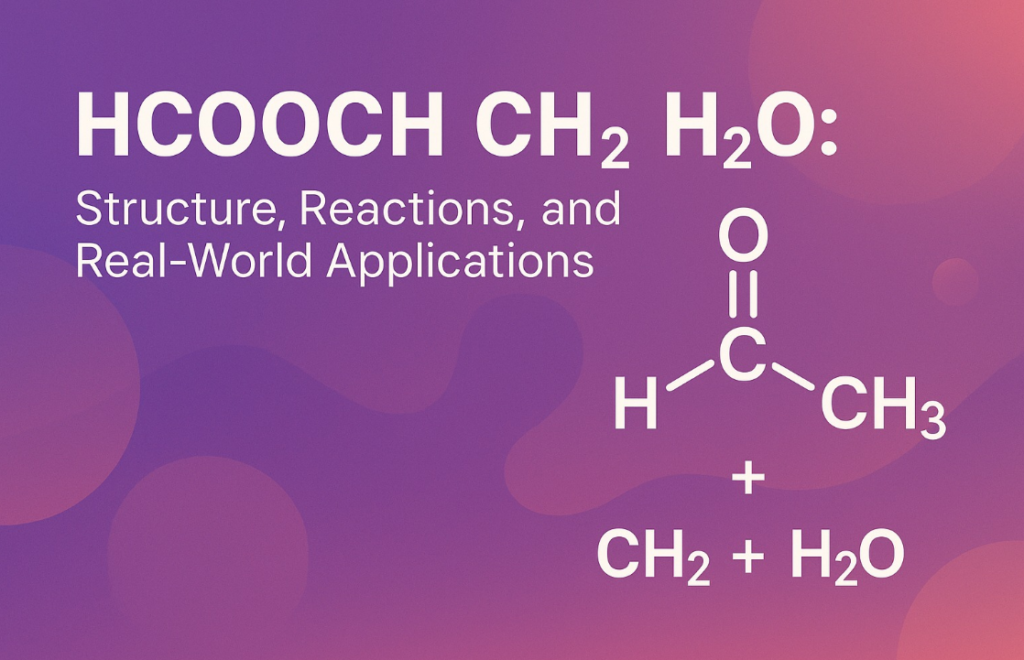
Chemistry plays a vital role in both our everyday lives and the industries that power our modern world. One chemical combination that often appears in academic discussions and industrial processes is the reaction involving formic acid (HCOOCH CH2 H2O), a methylene group (CH₂), and water (H₂O). While these molecules might sound complicated at first, they hold fascinating properties and real-life applications. This article aims to explain this reaction and its significance in simple English with a detailed look at the science, benefits, applications, and future.
What Is HCOOCH CH2 H2O All About?
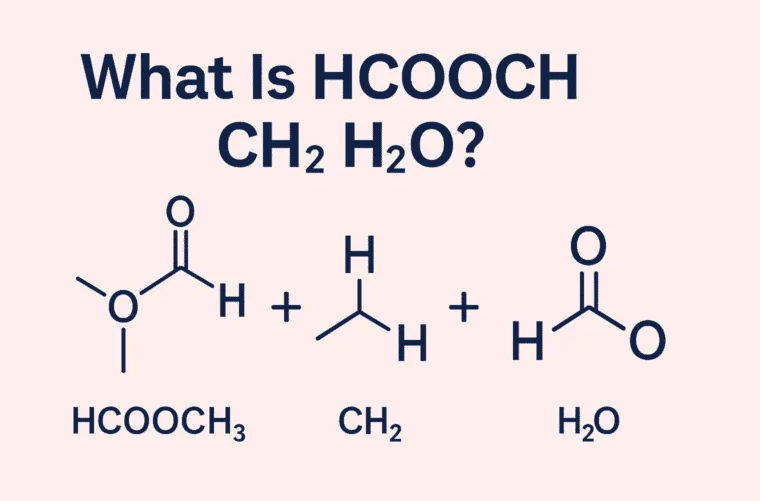
To begin with, it’s essential to break down the key players in this reaction. HCOOH is the chemical formula for formic acid, the simplest type of carboxylic acid. It’s naturally found in ant venom and is known for its strong smell and acidic nature. The methylene group (CH₂) is a part of many larger organic molecules and acts as a connector between different parts of a chemical structure. Water (H₂O), as we all know, is vital for life, but it also serves as a medium and sometimes a reactant in chemical reactions.
When we talk about the reaction between HCOOCH CH2 H2O, we are usually referring to a sequence of chemical transformations that can produce various useful compounds such as methyl formate, methanol, or carbon dioxide. These reactions are widely used in industries such as fuel production, food preservation, pharmaceuticals, and chemical engineering.
Formic Acid (HCOOH): A Closer Look
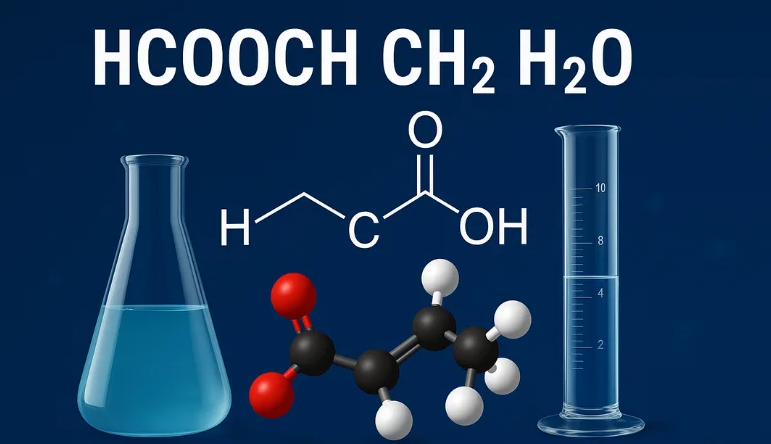
Formic acid is composed of just four atoms—one carbon, two oxygen, and two hydrogen. Its structure is simple but powerful. It belongs to a class of compounds known as carboxylic acids, and due to its small size, it reacts quickly and efficiently with other chemicals.
This acid is not only found in the wild—insect bites like those from ants and stinging nettles—but is also synthesized in factories for many industrial uses. It can act as a preservative, reducing agent, and intermediate in the manufacture of more complex molecules. In terms of reactivity, it is known to participate in esterification, oxidation, and reduction reactions, especially when combined with alcohols or metal ions.
Understanding CH₂: The Methylene Bridge

The methylene group, written as CH₂, is a very common building block in organic chemistry. Although it’s not stable on its own, it exists as part of larger molecules, acting like a bridge or connector between functional groups.
The role of CH₂ in reactions with HCOOH and H₂O is typically seen in intermediate states, especially when building larger organic compounds like esters or alcohols. In some cases, CH₂ may appear as part of formaldehyde, which is an industrially important molecule used in resins and plastics.
When CH₂ is present in a chemical reaction involving water and formic acid, it often contributes to the creation of chains or rings of carbon-based molecules, especially when the temperature or pH is controlled precisely.
Water (H₂O): More Than a Solvent
Water is not just a liquid we drink; in chemistry, it is one of the most important substances for initiating, supporting, and stabilizing reactions. In the case of the HCOOH and CH₂ combination, water can do many things.
Firstly, water can act as a medium, allowing ions and molecules to move around and react more efficiently. Secondly, it can participate directly in reactions such as hydrolysis, where water helps break bonds between atoms. Thirdly, it can affect the temperature and acidity (pH) of a reaction, both of which are crucial for making sure the chemical transformation goes in the right direction.
In a lab setting, water also helps dissolve ionic compounds, stabilizing their structures and making them more reactive.
How Does the Reaction Work?
When formic acid reacts with compounds containing CH₂ in the presence of water, various reactions can take place. One of the most common is esterification, where formic acid reacts with an alcohol to produce an ester (like methyl formate) and water.
Here is a simplified table showing potential outcomes:
| Reactants | Reaction Type | Products | Use |
|---|---|---|---|
| HCOOH + CH₃OH | Esterification | Methyl Formate + H₂O | Perfume industry, solvents |
| HCOOH + CH₂ (as CH₂O) | Oxidation | CO₂ + H₂O | Fuel cells, clean energy |
| HCOOH + CH₂Cl₂ + H₂O | Hydrolysis | Formaldehyde + Acid | Resins, plastics |
These reactions are usually exothermic, meaning they release heat. They are carefully controlled in industrial environments to maximize yield and ensure safety.
Where Do We Use This Reaction in Real Life?
This reaction is not just theoretical—it has many real-world uses. One major application is in fuel cells, where formic acid is used to release hydrogen gas in a clean and safe way. Another use is in the textile industry, where formic acid helps process leather and wool.
In the food industry, formic acid serves as a preservative to keep foods fresh and free from harmful bacteria. It is recognized for its biodegradable nature, making it safer than synthetic chemicals.
In pharmaceuticals, reactions involving HCOOH and CH₂ help in synthesizing complex organic drugs, especially antibiotics and painkillers. This reaction helps in forming carbon-based chains necessary for the active molecules in medicine.
Scientific Background: Reaction Types Involved
To fully understand the behavior of this combination, it’s helpful to explore the types of chemical reactions it may undergo.
Hydrolysis is a process where water breaks down a compound. In this case, if methyl formate is produced, it can later be broken down by water back into formic acid and methanol.
Esterification is the formation of an ester from an acid and an alcohol. This is common in perfume and flavor production.
Redox reactions involve the gain and loss of electrons. Formic acid is often used to reduce metal ions, making it important in chemical synthesis.
These reactions show how versatile this combination is, adapting to different needs based on the reaction environment.
Interesting Facts and History
Formic acid got its name from the Latin word formica, meaning ant, because it was first extracted by distilling ant bodies in the 17th century. It has been studied for centuries for its unique properties and continues to be relevant in modern research.
One fascinating historical use was during World War II, where formic acid was used in rubber production and for cleaning industrial equipment. Today, it’s being considered as a green hydrogen carrier in renewable energy technologies.
Applications in Research and Education
In academic settings, this reaction is used to demonstrate fundamental concepts in organic chemistry. Students often perform lab experiments that involve creating esters using formic acid and various alcohols. These experiments help explain concepts like equilibrium, catalysis, and thermodynamics.
Educational labs often use formic acid reactions to show how small changes in temperature or pH can dramatically affect the outcome of a chemical reaction.
Safety Considerations
Even though formic acid is naturally occurring, it is not without risks. It is highly corrosive and can cause burns if it comes into contact with the skin. Proper protective equipment such as gloves and goggles is essential when handling it.
Methylene-related compounds, especially formaldehyde or dichloromethane (which include CH₂ units), can be toxic if inhaled or ingested. Water, although safe, must be handled carefully in hot or pressurized conditions.
Always follow proper Material Safety Data Sheet (MSDS) guidelines when working with these chemicals in a lab or factory.
What Does the Future Hold?
The future of this reaction looks promising, especially in green chemistry. Researchers are exploring how formic acid can be used to store and transport hydrogen, which is a clean alternative to fossil fuels. CH₂-based compounds are being studied for their role in creating biodegradable plastics, which can help reduce pollution.
In the pharmaceutical world, modified versions of this reaction are being developed to create more efficient drug synthesis methods with fewer byproducts.
Conclusion: A Simple Reaction with Huge Possibilities
What may seem like a simple combination—HCOOCH CH2 H2O—actually represents a complex and powerful set of reactions that touch almost every part of our lives. From the food we eat to the fuel we use, these molecules help drive progress in chemistry, industry, and environmental safety.
Whether you’re a student, a scientist, or simply a curious reader, understanding how these components interact can offer a deeper appreciation for the beautiful complexity of chemistry. This reaction shows how small, invisible molecules can have visible, life-changing impacts.
We encourage you to contact us.